The C-cl Bond Is Best Described as
CCl4 Carbon tetrachloride is nonpolar in nature. The C-Cl bond has a large difference in electronegativity compared to the H-C bonds.
Have high melting points B.

. H and Cl 8. No dipole D polar. The C-Cl bond is best described as Nonpolar covalent Polar covalent Ionic O Coordinate covalent O None of these Identify the relationship between the following two structures.
The lone pair on chlorine are dispersed throughout the benzene ring by resonance. Which one of the following compound is most likely to be an ionic compound. C Cl 2 and Cl 2.
HH cic C- H HHHH A polar. Contains only ionic bonds solid A contains only ionic bonds and solid B contains only covalent bonds 5. ОН ОН ОН В.
AIt contains ionic bonds and has a low melting point. The polar groups of the soaps form the surface of the micelle and hydrogen bond to the surrounding water. Video Explanation Solve any question of Haloalkanes and Haloarenes with- Patterns of problems.
Four electrons are shared. The C-Cl bond is best described as A. H 2 Cl 2 2HCl Which of the following statements best describes in this reaction.
Cl Mg 68. 1 point a 368 kJ. 6 Given the reaction.
Write a Lewis structure for the chlorate ion ClO. Although the four bonds C-Cl are polar because of the difference in electronegativity of Chlorine 316 and Carbon 255 CCl4 is nonpolar because the bond polarity gets canceled with each other due to the symmetrical geometrical structure tetrahedral of the CCl4 molecule. A The forming of the H-Cl bond releases energy b The forming of the H-Cl bond absorbs energy c The breaking of the H-H bond releases energy d The breaking of the Cl-Cl bond releases energy.
Al K c. Which of the following statements best describes the C-Cl bond in the following compound. And a melting point of -967 C.
Bonds that have an even distribution of charge. DIt contains covalent bonds and has a high melting point. 1- Use the reaction to complete the sentence.
22Which statement best describes the substance that results when electrons are transferred from a metal to a nonmetal. The Cl Cl bond is best described as A nonpolar covalent B polar covalent C ionic from CHM 241 at Northern Virginia Community College. Soaps form clusters called micelles.
Covalent because valence electrons are shared B. According to the VSEPR theory the molecular geometry of ammonia is. 8 at carbon and S-at chlorine B ionic C nonpolar.
Describe how soaps function as cleaning agents. CH2Cl2 Lewis Structure Molecular Geometry Hybridization and MO Diagram. The C-Cl bond in the butyl chloride CH 3 -CH 2 -CH 2 -CH 2 -Cl is polarized due to electronegativity difference.
C S d. 50 The C Cl bond is best described as A Nonpolar covalent B Polar covalent C from CHEM 200L at University of Santo Tomas. Have low boiling points.
The electrons are withdrawn by the chlorine atom. P and Cl D. It is polar because of the presence of two chloro groups but is not.
H2 Cl 2 2HCl Which statement best describes the energy change as bonds are formed and broken in this reaction. Thus the first carbon atom gets partial positive charge. The total bond energy of the products is 1472 kJ.
CH3Cl is always polar. 15The bonds in BaO are best described as Aan ionic solid Ba network solid Ca metallic solid Da molecular solid 16A substance that has a melting point of 1074 K conducts electricity when dissolved in water but does not conduct electricity in the solid phase. An ionic bond is best described as A the sharing of electrons.
B the transfer of electrons from one atom to another. Carbon monoxide and oxygen combine to produce carbon dioxide. What statement best describes the polarity of CH3Cl.
The substance is most likely ACH3OH BC6H12O6 CH2O DKOH. 2CO O2 2CO2. 33 Given the reaction.
The bonds in BaO are best described as A. Between 100C and 900C. The data shows bond fission leading to the production of ground-state.
Molecule is best described as A linear. That work evidenced two competing C-Cl bond fission channels tentatively assigning them as producing ground- and excited-state methoxy carbonyl radicals. A Two electrons are shared.
-at carbon and 8 at chlorine E None of these. C the attraction that holds the atoms together in a polyatomic ion. Option C is correct.
This gives the CCl bond a double bond character. The CCl bond in C 6 H 5 Cl is shorter than in CH 3 Cl and therefore stronger. C Two electrons are transferred.
CH3Cl is a fairly polar molecule. CIt contains covalent bonds and has a low melting point. 4 The water loses heat and the block gains heat until both are at the same temperature between 100C and 900C.
In turn this carbon atom drags electron density partially from the next carbon which also gets partial positive charge. Which statement best describes how a catalyst can speed up a chemical reaction. The interior of the micelle is composed of the nonpolar hydrophobic portions of the soap molecules.
A characteristic of ionic solids is that they A. TRUEFALSE The bond in F 2 is described as polar covalent. In this study we measure the photofragment angular distributions for each C-Cl bond fission channel and the spin-orbit state of the Cl atoms produced.
The bond energy of each carbon-oxygen bond in carbon dioxide is _____. Dichloromethane or methylene chloride with the chemical formula CH2Cl2 is a colorless volatile liquid with a boiling point of 396 C. Which statement describes a multiple covalent bond.
Carbon Tetrachloride is an IUPAC. It is widely used as a solvent in chemistry laboratories. D the attraction between two nonmetal atoms.
BIt contains ionic bonds and has a high melting point. Which formula represents a mo ecu ar compound. D LiCl C KCI 6.
Which of the following statements best describes the C-Cl bond in the following compound. D Four electrons are transferred. Grease and dirt are nonpolar and have limited.
OH OH Enantiomers Identical Neither Which of the following is the correct structure for the compound R-2-pentanol. 1 breaking HH bonds releases energy 2 forming HCl bonds absorbs energy.

Che 140 Ch 8 Learn Smart Flashcards Quizlet

Final 21 December 2018 Questions And Answers Chapter 6 Financial Assets Quiz It Is A Report That Studocu

The Bond Angle Of H 2 Se Is Best Described As

Thalassa Goa Goa Beach Shacks Top 10 Restaurants

Bond Polarity Flashcards Quizlet

Solved An Ionic Bond Is Best Described As A The Attraction Chegg Com

Organic Chemistry 331 Sapling Learning Ch 4 Flashcards Practice Test Quizlet

Chemistry Chap 4 Flashcards Quizlet

Covalent Bonds Flashcards Quizlet

All About Your Life Top 3 Places To Shop For Online Jewelry Jewelry Online Shopping Amazing Jewelry Beautiful Jewelry

This Is Sodium Chloride It Reminds Me Of Chemistry And Everything That Came With That Class Ionic Compound Extracellular Fluid Chemistry
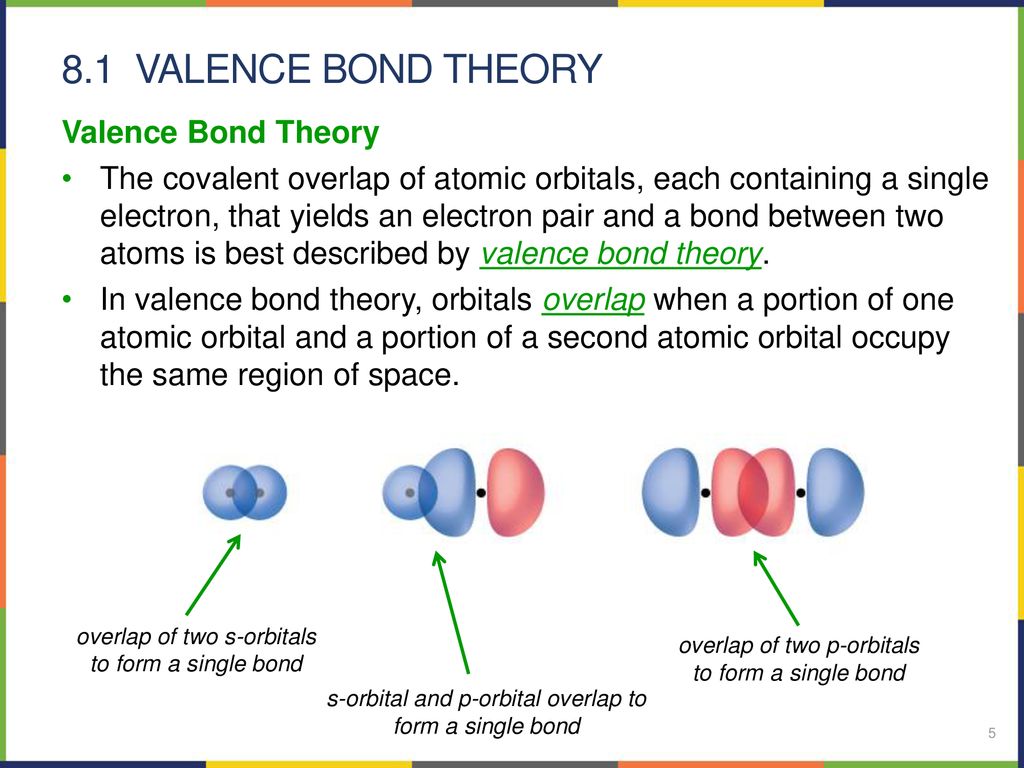
Chemistry Advanced Theories Of Covalent Bonding Chapter 8 Ppt Download

Do Now Which Substance Contains Bonds That Involved The Transfer Of Electrons From One Atom To Another Co2 Nh3 Kbr Cl2 When Sodium And Fluorine Combine Ppt Video Online Download
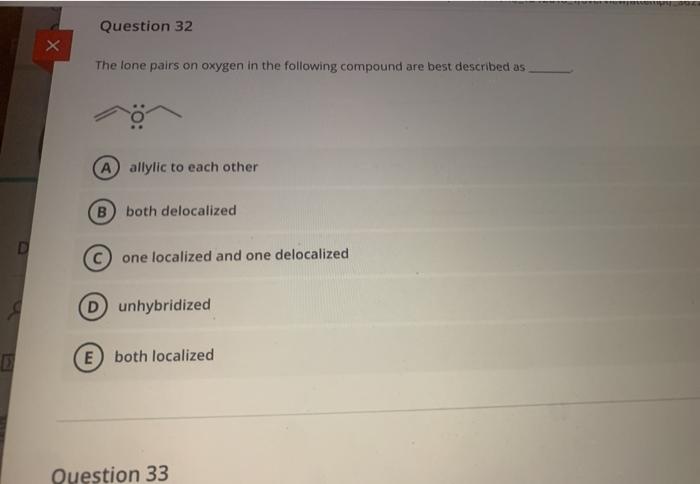
Solved Question 28 Identify The Resonance Structure Chegg Com

1 Structure And Bonding Why This Chapter Ppt Download

Organometallic Compounds Ppt Download

Organic Chemistry 331 Sapling Learning Ch 3 Flashcards Quizlet



Comments
Post a Comment